Soil testing the correct way and why it matters
Soil testing the correct way and why it matters
Gardeners, unlike farmers, are not required by law to regularly test their soil, but it’s a good idea to test your soil’s pH at the very least. Soil pH directly affects the availability of nutrients in the soil for your plants to use. By understanding this relationship, you can make informed choices with regards to effective soil maintenance and create the best growing conditions for your plants.
By knowing your soil pH, you can confidently buy plants that are known to thrive in your garden conditions and can avoid costly mistakes such as planting calcifuges in alkaline soil. In the wrong pH they will struggle to establish, suffer from nutrient deficiencies and eventually die. As in all gardening you should always aim to stick with the adage ‘Right Plant, Right Place’.
You can improve your soil texture and structure by increasing its organic material percentage. You can also break up any pans thus improving drainage, aeration and the overall ease that plants can root down into the soil. A pan is an area of soil, usually below the surface, that is so compacted that it will not drain effectively and plants would struggle to get any roots though the affected area. However, trying to reduce your soil pH is a fool’s errand and is, in the long term a waste of effort. Raising your pH is more doable, but I would always recommend that you just use plants that are appropriate for your soil.
Selecting plants that are suited to you soil type will also mean that you need to feed far less if at all. The over use of fertilisers is responsible for huge amounts of pollution running off the land, into our watercourses and eventually into the sea. This causes the acidification of the sea and is responsible for much loss of habitat including the wholesale demise of huge swathes of corals in the Great Barrier Reef and around the world.
How to test your soil
Basic soil pH testing kits are freely available in garden centres and on the internet. They range in price from approx. £2.99 to £15.99 and can be used from 3 to 15 times. These kits are designed for home use and are straight forward and safe. It’s a good way to get children involved in gardening as they seem to love the little test tubes and processes involved. There are also soil testing strips but I have not used them and cannot claim to know their efficacy.
Do not waste your money on expensive probes or meters as these cannot be calibrated and can therefore be quite inaccurate.
The kits contain an empty test tube, a small measuring spoon and a pH guide with instructions and two basic ingredients, diluted Potassium chloride indicator in liquid form and Barium sulphate as a powder.
The Barium sulphate acts as a flocculant, which binds the soil particles in the liquid so that they sink to the bottom of the tube, thus allowing you to get a clear reading from the colour of the liquid. The Potassium chloride reacts with the soil and changes colour to indicate the overall acidity of the sample. Because potassium fertilisers rarely have any effect on pH this produces a pretty accurate result.
Taking a soil sample
This is the bit that is easiest to mess up, but a few sensible precautions will keep you on track. Firstly, wear gloves and do not touch the soil sample. The oils in our skin are acidic and can affect the outcome of the test. The soil samples are tiny and should be taken from approximately 10-15cms below the surface. Testing at this depth, means you are testing the top soil and not the organic layer on the surface, which could contain leaves, other organic material, compost and substances that will affect the result. Do not take samples from areas that have had imported top soil incorporated. The results will be useless. Do your test in areas that have not had any topsoil added. Use plastic tools to take the sample and make sure they are clean before you start. Metal tools should be avoided. Take more than one sample from each area you are testing. This is to allow you to get an average reading from that particular bed or area. Some gardens have different pH readings due to changing geological features beneath the soil. This can mean that some areas can support different plants. This can happen even in relatively small gardens.
Taking multiple small samples in zigzag pattern in each bed is a good way to get a reliable average reading.
The small samples are then mixed together and a specimen amount taken and tested. Some testing kits require the soil be dried before testing. If you need to do this, ensure the sample dries naturally on a clean ceramic plate or plastic tray. Don’t dry it on paper or newspaper, both of which can be acidic and never dry the soil in an oven or microwave as some soils can contain traces of metals and could explode.
Once you have your prepared sample, take the prescribed amount and place it in the clean test tube. A small amount of barium sulphate powder is added and then the testing solution poured on top to a specified line on the test tube. The instructions stating the amounts for each of the ingredients should be closely followed. Seal the test tube and shake it all together until it is properly mixed. The test tube is then set down in an upright position and left for approx. 10 minutes to settle.
Each test kit has a colour guide included and this should be taken outside into natural light and compared side by side with the liquid in the tube. (Don’t check this under electric light as it can affect the accuracy of the reading. Most man-made light has a sulphurous yellow tone and LEDs can be either red or more likely blue toned.)
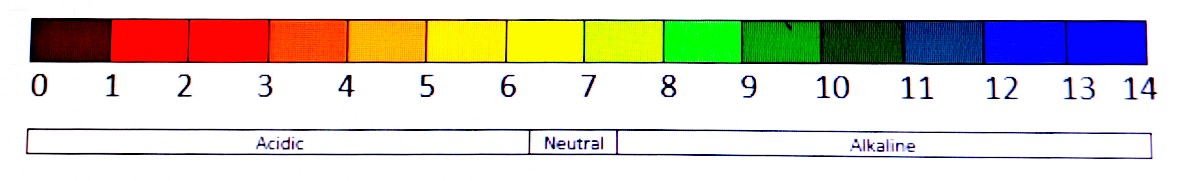
The colours indicate the acidity of the soil sample and are arranged in a logarithmic scale. This means that a reading of 7 is 10 times less acidic than a reading of 6 or more alkaline if you prefer, and a reading of 8 is 100 times more alkaline than a reading of 6. It is likely that your reading could fall somewhere between two colours on the scale so you would divide the space between the two colours into 10 and take your reading accordingly. So, if your reading is halfway between 6 and 7 it would be a reading of 6.5 etc.
It is useful to record each pH reading on a birds-eye sketch of your garden for future reference.
Some kits also come with a useful guide which shows the main groups of plants that are best suited to particular pH ranges. On average 7 is considered neutral however this is not always entirely accurate as temperature and other factors can affect this. For the purposes of gardening, a sample showing a reading of 6.5 – 7.3 is considered neutral. Lower numbers are acidic and higher numbers are alkaline. If you have very high or low reading this could indicate that the area is polluted. For example, a reading of 3 is strongly acidic like vinegar and would be injurious to any plant. Similarly, a reading of 10 is strongly alkaline and could behave in the same way as bleach and should be investigated by a professional.
In areas of higher rainfall, the natural pH of soil typically ranges from 5 to 7, while in drier areas the range is 6.5 to 9.
Neutral soils have the highest amounts of nutrients available for take up by the plants, so those of you lucky enough to have this could probably grow the largest range of plants. Both, neutral and acidic soils have Iron and Magnesium freely available to (calcifuges), acid loving or ericaceous plants. In alkaline soils these nutrients are unable to be accessed and utilised by these kinds of plants and they suffer badly.
Alkaline or basic soils with a pH of 7.4 and above have higher levels of calcium so they are better suited to lime loving plants (calcicoles).
|
Plants requiring or tolerant of neutral to acidic soil |
Plants requiring or tolerant of alkaline soil |
|
Azalea |
Achillea |
|
Blue flowered Hydrangea macrophylla* |
Albizzia* |
|
Camellia |
Amelanchier* |
|
Cornus* |
Asclepias |
|
Lamprocampnos/Dicentra* |
Astilbe |
|
Most Erica |
Berberis |
|
Calluna |
Brunnera |
|
Enkianthus |
Campanula |
|
Crinodendron |
Caryopteris |
|
Liriope* |
Ceanothus* |
|
Acer (Japanese Maples) |
Clematis |
|
Beech* |
Convallaria |
|
Cedar* |
Cotinus |
|
Pieris |
Crataegus |
|
Pinus |
Dianthus |
|
Picea* |
Geranium |
|
Sorbus* |
Hosta |
|
Salix* |
Juniperus |
|
Gardenia |
Lavender |
|
Rhododendron |
Lonicera (Honeysuckle) |
|
Magnolia* |
Origanum |
|
Glandora (Lithodora)* |
Phlox |
|
Anemone hybrida* |
Polemonium |
|
Digitalis* |
Primula |
|
Blechnum |
Salvia |
|
Athyrium* |
Syringa (Lilac) |
|
Vaccinum (Blueberry) |
Thymus |
The table shows just some of the plants that can thrive in the different pH ranges. Plants marked with an asterisk are also able to thrive in other conditions.
Many plants have little preference either way and will survive in pH ranges from 5 to 9, so you will always find something beautiful that loves your soil. Now all you have to do is check the other conditions to confirm that the moisture, light and exposure levels are to the plant’s liking.
In summary, Easy and safe to use, home testing pH kits can help you understand your soil and the kinds of plants that will thrive and those that will not. Spending a few moments conducting pH tests in the correct manner can help you buy with confidence, reduce plant failures and the need for fertilizers, which is kinder to the plants, you and the planet.
